Great things take time: the world of medicine breakthroughs

My mentor advised me to read this book called Breakthrough: The quest for life-changing medicines. I did read it, and I loved it. It is not only insightful and knowledgeable, but it is also an eye opener. I did always know that drug discovery is very difficult with years going into invention, and even post invention, most of the drugs do not get approved for clinical usage. However, reading actual stories of the drugs known to the humankind is mind boggling. It makes you realize that good things, if planned correctly, might occur as per the plan.
However, great things - they take time, blood, sweat, patience, resilience and acceptance of failures. Real innovation is not that easy.
Another reason I loved the book is that it made me feel proud. I feel very proud to be associated to this amazing domain: healthcare and pharmaceuticals. The quest for medicine innovation is remarkably complicated. When I usually looked at some of the clinical trial results before, I used to feel that something might have gone wrong or it could have been planned differently. Post reading this book, my perspective has changed. Look at the statistics: most of the drugs never see the light of the day because they fail even before clinical trials. The ones which do reach clinical trials - there is a 90%+ chance of failure. The author clearly explains why is that - by drawing parallels to the game of chess. Chess is one of the most complicated games in the world with more than 10^40 different moves possible. However, human body is so much more complicated with each of us having more than 30-40 trillion cells in our body.
Moreover, each cell is composed of molecules and each human body is also different. To find which drug would work for us is infinitely more complex than a game of chess. That is the reason it has taken decades for us to reach inventions such as Paracetamol, or a reliable HIV management and prevention drug.
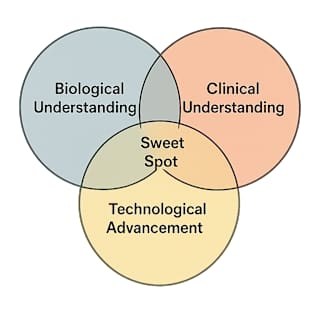
According to the author, the successful innovation in drug discovery is a combination of three factors - biological understanding, clinical understanding and technological advancements. There needs to be a sweet spot hitting all the three factors for the successful drug to be launched. William Pao, the author, also shares stories of some of the most transformative drug innovations ever launched in the history, which are essentially "Breakthroughs".
After reading each of the story, there is one thing that I found common - failures.
It does sound cliche, however, I actually found that one way or another, the lead researchers, scientist, pharma company backing the innovation somehow lost faith in between because of years of work but no results. However, there came that Eureka moment, which led to the discovery of that one final molecule that would work. I really suggest this book if you are interested in knowing how these breakthroughs took place - its an amazing read.
Below I have summarized the top breakthroughs which are described at length in the book. I have simply and briefly summarized these stories (taking inspiration from the book and publicly available knowledge). I am particularly really proud of the last one because I happen to work at the company who did that.
💊 Paracetamol: A Cure Born from a Mistake
In the 1880s, two German doctors intended to treat a patient’s intestinal worms with naphthalene. But due to a pharmacy error, the patient received acetanilide instead. It didn’t kill the worms — but it lowered the fever.
That mistake sparked interest. Scientists explored acetanilide, only to find it was toxic to red blood cells. This failure led to phenacetin, which was later found to break down into paracetamol — a compound discovered in 1893 but overlooked for decades.
It took until the 1950s, and years of questioning old assumptions, to realize paracetamol was the real hero all along — a safer, effective painkiller born out of randomness, error, and persistence.
💊 Osimertinib: Solving the Mystery of Cancer’s Comeback
In the early 2000s, lung cancer doctors had reason to hope. A new class of drugs showed promise for patients with specific gene mutations. Tumors shrank. Lives were extended. But the hope didn’t last.
After a few months, the cancer came roaring back.
Doctors were puzzled — why were patients relapsing so quickly? It turned out the cancer was fighting back with a sneaky mutation called T790M, making existing drugs useless. It was like hitting a moving target that always stayed one step ahead.
At AstraZeneca, a small team took on the challenge. They weren’t sure if a solution even existed. They sifted through compound after compound, tweaking molecules, testing ideas, facing failure after failure. Still, they pushed forward.
Then they found it — a molecule that didn’t just work, but worked brilliantly. It could target the mutated cancer without harming healthy cells. The drug was called osimertinib (later branded as Tagrisso).
By 2015, it was approved and saving lives — especially for those who had run out of options.
💊 Alpelisib: Cracking Cancer’s Genetic Code
For years, some breast cancers kept coming back, defying hormone therapy. The reason? A hidden genetic glitch — a mutation in the PIK3CA gene — quietly fueling resistance in nearly 40% of patients.
Drug developers tried to shut it down, but early treatments caused too many side effects or didn’t work well. Just when it seemed hopeless, scientists designed a smarter solution: alpelisib — a drug that precisely targeted the mutation.
In trials, pairing alpelisib with hormone therapy nearly doubled the time before cancer returned. In 2019, it became the first approved PI3K inhibitor for breast cancer — a breakthrough that turned a deadly mutation into a treatable weakness.

💊 Nirmatrelvir: A Drug Decades in the Making
When COVID-19 hit in 2020, the world scrambled for vaccines — but what about pills to treat it?
At Pfizer, scientists dusted off an old idea: a compound originally designed after the 2003 SARS outbreak but never used. That compound became the starting point for nirmatrelvir, tweaked to block the virus’s key enzyme from replicating.
They moved fast — from lab bench to approval in under two years. In clinical trials, Paxlovid (nirmatrelvir + ritonavir) slashed hospitalizations and deaths in high-risk patients by nearly 90%.
Approved in late 2021, it became the first widely available oral antiviral for COVID-19 — a lifeline born from speed, science, and a shelved idea that suddenly found its moment.
💊 CTX001: Editing Out Sickle Cell Disease
For decades, sickle cell disease meant a lifetime of pain and limited options. But scientists noticed something curious: some patients with high levels of fetal hemoglobin stayed healthier.
They traced this back to a gene called BCL11A, which shuts off fetal hemoglobin after birth. What if they could just switch it back on?
Using CRISPR, scientists did exactly that — editing patients’ own cells to reactivate fetal hemoglobin. The result: CTX001, later named Casgevy.
In 2023, it became the first FDA-approved CRISPR therapy, offering a potential cure — not just treatment — for sickle cell disease.
A lifelong illness, now rewritten at its genetic root.

💊 Lenacapavir: A Breakthrough in the Fight Against HIV
For years, researchers struggled with HIV’s ability to quickly develop resistance to treatments. But then came lenacapavir — a first-in-class drug that works by disrupting the HIV virus at its core: the capsid. This groundbreaking approach prevents the virus from replicating and mutating, offering a powerful new weapon in the fight against HIV.
Discovered by Gilead Sciences, lenacapavir was designed to target HIV in a way no other drug could. After years of testing, it was approved by the FDA in 2022 for people with multidrug-resistant HIV, offering a long-acting option with just two doses per year.
What makes lenacapavir even more exciting? It’s shown 100% effectiveness in preventing HIV transmission in trials, especially in high-risk groups. It’s a game-changer, with the potential to prevent new infections and offer a lifeline to those who have run out of treatment options.
Lenacapavir is revolutionizing both HIV treatment and prevention, bringing us one step closer to a world without HIV.
There are more such stories shared in the book, but I just wanted to give an idea, and pique your interest.
These stories prove that 'great things take time'.
So whichever field we are pursuing, real breakthroughs would need a lot of commitment, resilience, and grit. The book sheds perseverance all through the stories of the major drug discoveries and I am deeply inspired.
I do feel that the time to discover the drug will reduce significantly with the technological advancements leading to mapping out the right molecule among millions or billions of molecule and that can unfold so many mysteries and improve patient outcomes. In fact, it will be an even a bigger breakthrough since it will be composed of other breakthroughs.
At the end, this quest is for the improvement of people's lives. Hence, there could not have been a bigger breakthrough.
References:


